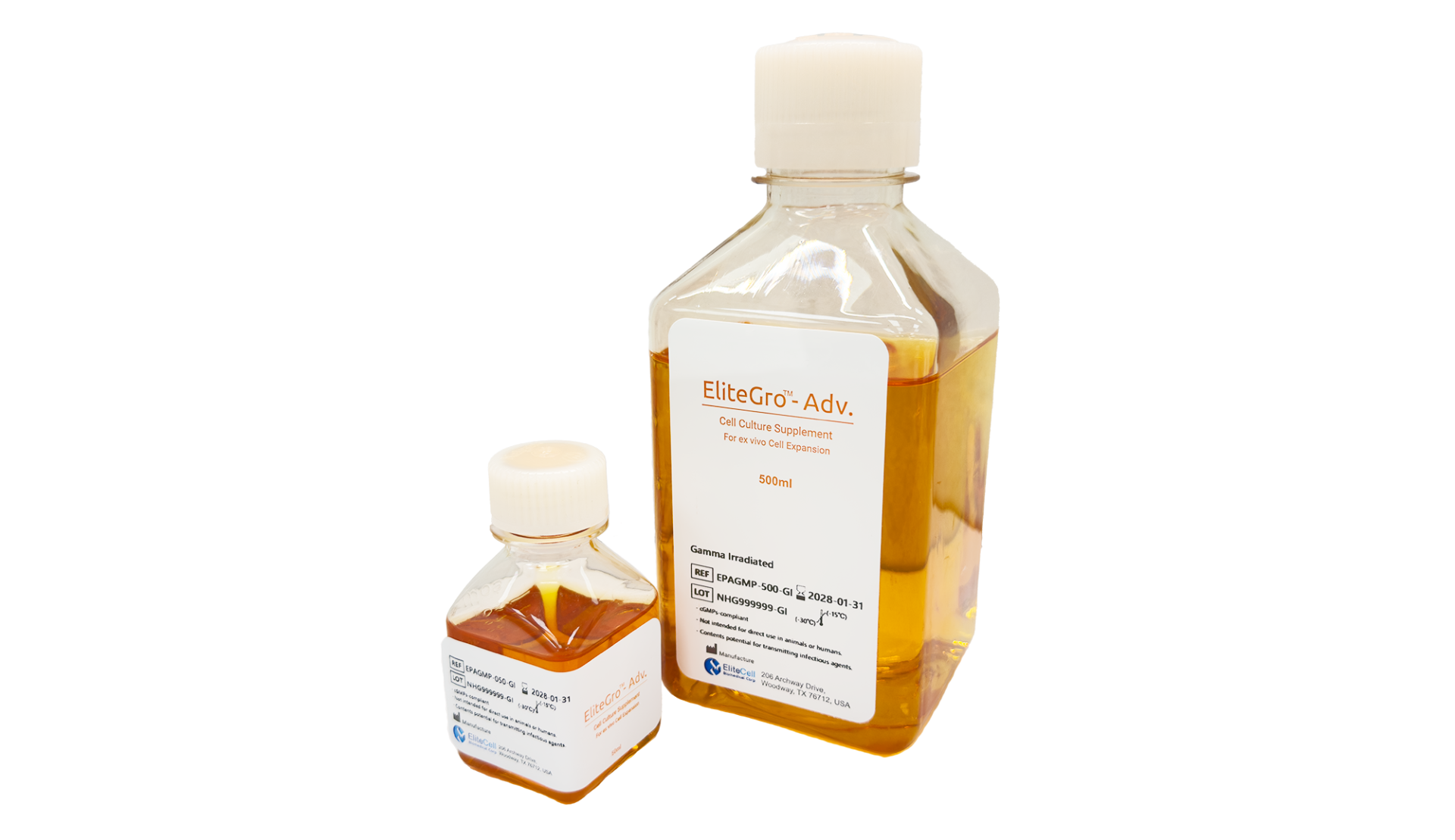
EliteGro™-Adv.-Gamma Irradiated (GI)
ID:EPAGMP-050-GI / EPAGMP-500-GI
EliteGro™-Adv.-GI is specially formulated to mitigate concerns about potential contamination, even at levels below detection limits. It undergoes gamma irradiation to effectively reduce pathogen presence and inactivate viruses, while maintaining its full performance and structural integrity.
50ml / EPAGMP-050-GI
500ml / EPAGMP-500-GI
100ml / EPAGMP-100-GIBG
500ml / EPAGMP-500-GIBG
50ml / EPAGMP-050-GI
500ml / EPAGMP-500-GI
100ml / EPAGMP-100-GIBG
500ml / EPAGMP-500-GIBG
Why Choose EliteGro™-Adv.-Gamma Irradiated (GI)?
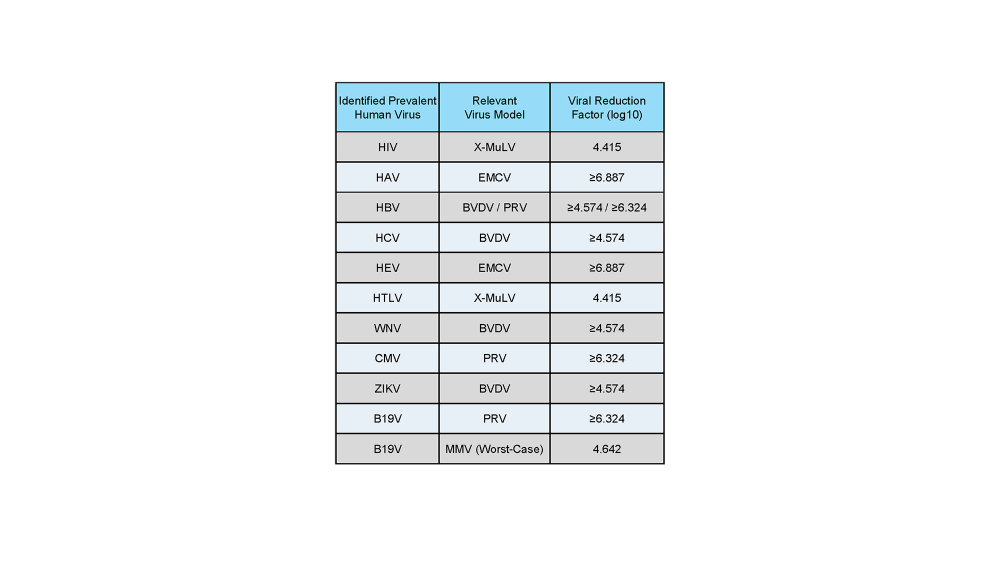
EliteGro™-Adv.-GI is tailored for clinical therapeutic cell development, where safety and consistency are critical. It undergoes a validated gamma irradiation process, in accordance with ICH Q5A guidelines, to effectively inactivate human infectious viruses—including B19 (Parvoviridae)—without compromising product integrity.
EliteGro™-Adv.-GI is tailored for clinical therapeutic cell development, where safety and consistency are critical. It undergoes a validated gamma irradiation process, in accordance with ICH Q5A guidelines, to effectively inactivate human infectious viruses—including B19 (Parvoviridae)—without compromising product integrity.

What’s the difference?
EliteGro™-Adv.-GI is the virus-inactivated counterpart of EliteGro™-Adv., produced using the same raw materials and manufacturing process to ensure equivalent performance and consistency. While all components in EliteGro™-Adv. are screened and confirmed to be free of transfusion-transmissible human viruses, EliteGro™-Adv.-GI undergoes an additional gamma irradiation step to further inactivate potential pathogens and viruses—providing an added layer of safety.
The formulation contains no heparin, anticoagulants, or animal-derived components, and features a clear, clot-free appearance optimized for cell culture. EliteGro™-Adv.-GI is available exclusively under cGMP-compliant environment manufacturing for use in clinical cell therapy development where the highest safety standards are required.
EliteGro™-Adv.-GI is the virus-inactivated counterpart of EliteGro™-Adv., produced using the same raw materials and manufacturing process to ensure equivalent performance and consistency. While all components in EliteGro™-Adv. are screened and confirmed to be free of transfusion-transmissible human viruses, EliteGro™-Adv.-GI undergoes an additional gamma irradiation step to further inactivate potential pathogens and viruses—providing an added layer of safety.
The formulation contains no heparin, anticoagulants, or animal-derived components, and features a clear, clot-free appearance optimized for cell culture. EliteGro™-Adv.-GI is available exclusively under cGMP-compliant environment manufacturing for use in clinical cell therapy development where the highest safety standards are required.
Performance
- Performance unaffected after Gamma Irradiation process.
- Support cell proliferation of multiple types of mesenchymal stem cells and immune cells without morphology or characterization change.
- Less cell doubling time, high cell growth rate.
- Seed in low density.
- Support cell proliferation of human lymphocytes (T cell, DC, CIK, NK cells, etc).

Advantage
- EliteGro™-Adv.-GI undergoes gamma irradiation for effective inactivation of human infectious viruses, including B19 (Parvoviridae), in accordance with ICH Q5A guidelines.
- Lot-to-lot consistency is ensured as EliteGro™-Adv.-GI is manufactured using the same raw materials and production process as EliteGro™-Adv., resulting in equivalent product performance.
- No heparin required, No animal material, no anti-coagulations used in EliteGro™-Adv.-GI.
- Stable and safe supply from US FDA registered and AABB accredited sources.
- Product was manufactured under cGMP-compliance environment.
-
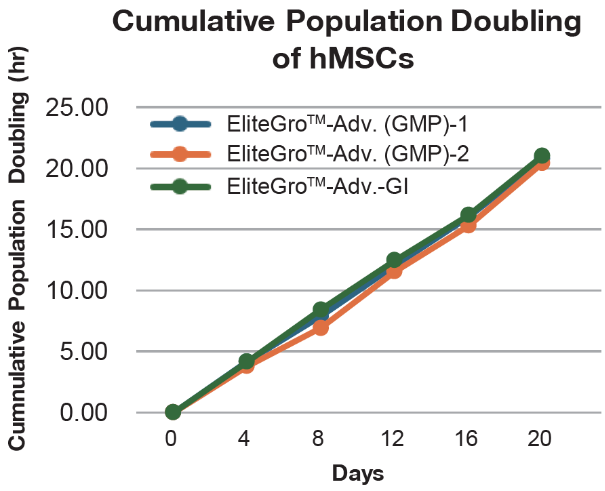
Comparison of EliteGro™-Adv.-GI vs EliteGro™-Adv. (GMP) for even better cell growth. (Human Mesenchymal Stromal Cells)
-
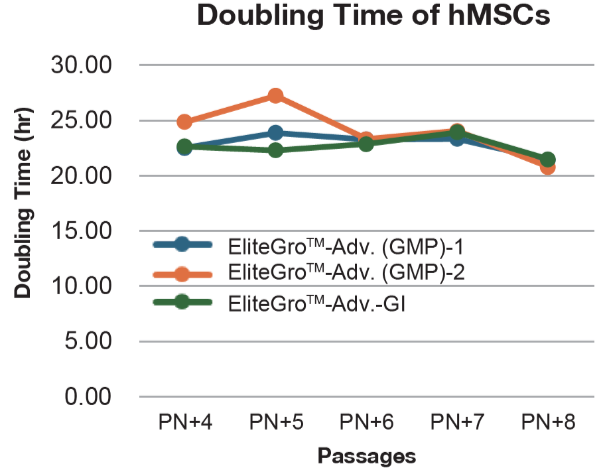 Comparison of EliteGro™-Adv.-GI vs EliteGro™-Adv. (GMP) to 8 passages.
Comparison of EliteGro™-Adv.-GI vs EliteGro™-Adv. (GMP) to 8 passages.
(Human Mesenchymal Stromal Cells)
Differentiation ability of hMSCs
-
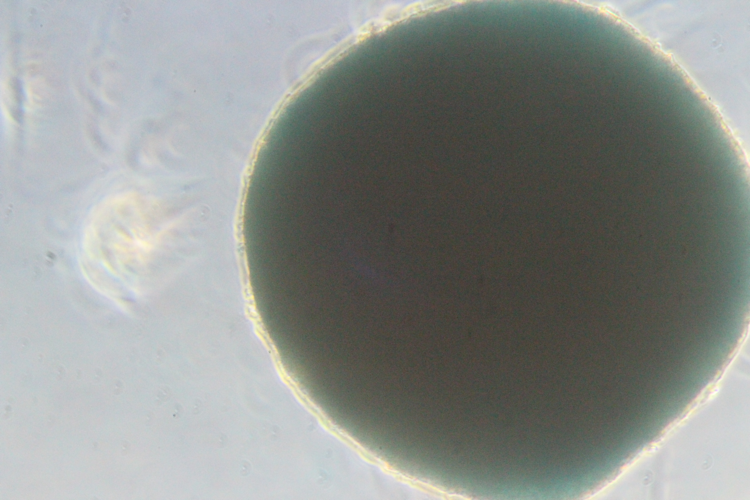 Chondrocytes
Chondrocytes -
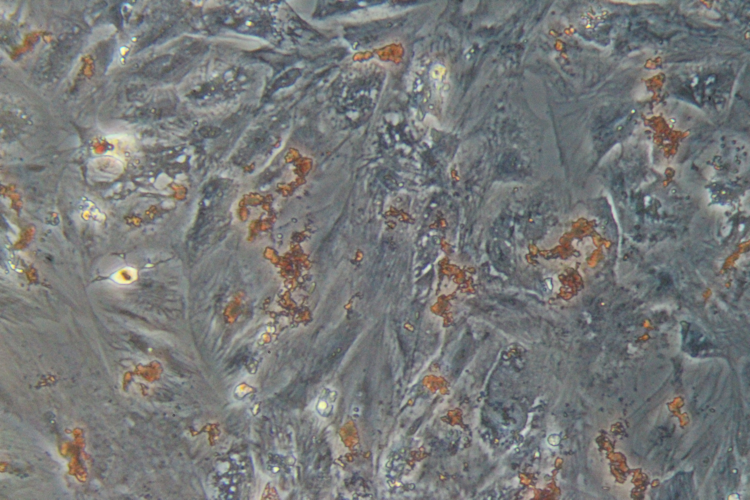 Adipocytes
Adipocytes -
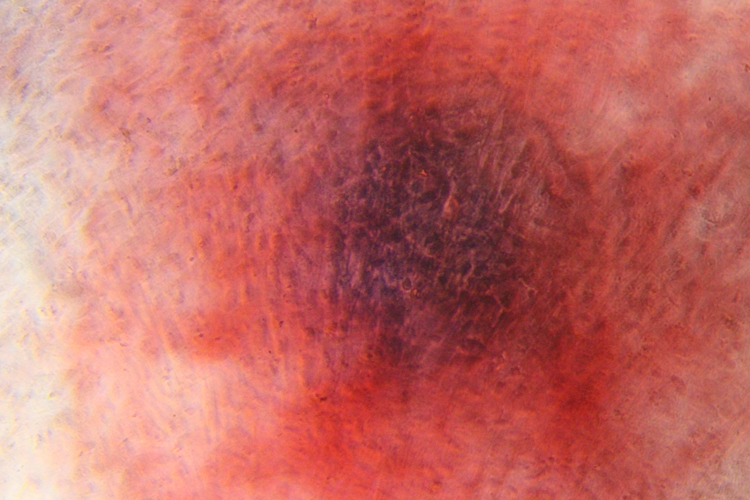 Osteocytes
Osteocytes